Architecture and device agnostic

Creating reliable and safe medical devices is critical for the medical industry. Embedded systems serve as the backbone for many life-saving devices and critical healthcare solutions, enabling real-time monitoring, diagnostics, and control. These systems power everything from pacemakers and infusion pumps to imaging equipment and wearable health devices. Embedded systems also drive new possibilities for remote care, telemedicine, and personalized health solutions, all while maintaining high standards of functional safety and regulatory compliance.
Our comprehensive range of solutions is designed to address this complexity effectively. With our development toolchain, we offer powerful code optimizations and rigorous code quality control, ensuring that your applications perform at their best while remaining robust.
Our tools are certified for functional safety according to ten different standards, including IEC 62304. This certification empowers you to develop compliant and secure medical-embedded applications with confidence.
The platform for embedded development
Scale development operations with freedom and flexibility, accelerate innovation with code confidence and simplify compliance while strengthening security.

Architecture and device agnostic

Cloud-ready, tailored for enterprises

Functional safety always included

End-to-end embedded security
Solutions
Compliance

As embedded systems grow more complex, functional safety becomes crucial, especially in industries like automotive where compliance is mandatory.
Our functional safety editions are TÜV SÜD-certified, ensuring they meet the necessary standards for developing safety-critical applications. By choosing IAR as your development platform, you eliminate the need to independently evaluate the tool’s development process or conduct your own compliance testing. TÜV SÜD has already handled this, saving you both time and costs, so you can focus on your code and application features.
Functional safety
The international standard IEC 62304 specifies life cycle requirements for the development of medical software and software within medical devices.
| Arm | Risc-v | Renesas RL78 | Renesas RX | Renesas RH850 | STM8 | |
| Medical IEC 62304 |
Get inspiration on how world-leading companies enhance their embedded development with our solutions.

Improve the speed of innovative development used to develop advanced medical devices with IAR's platform like Osong medical innovations have done.

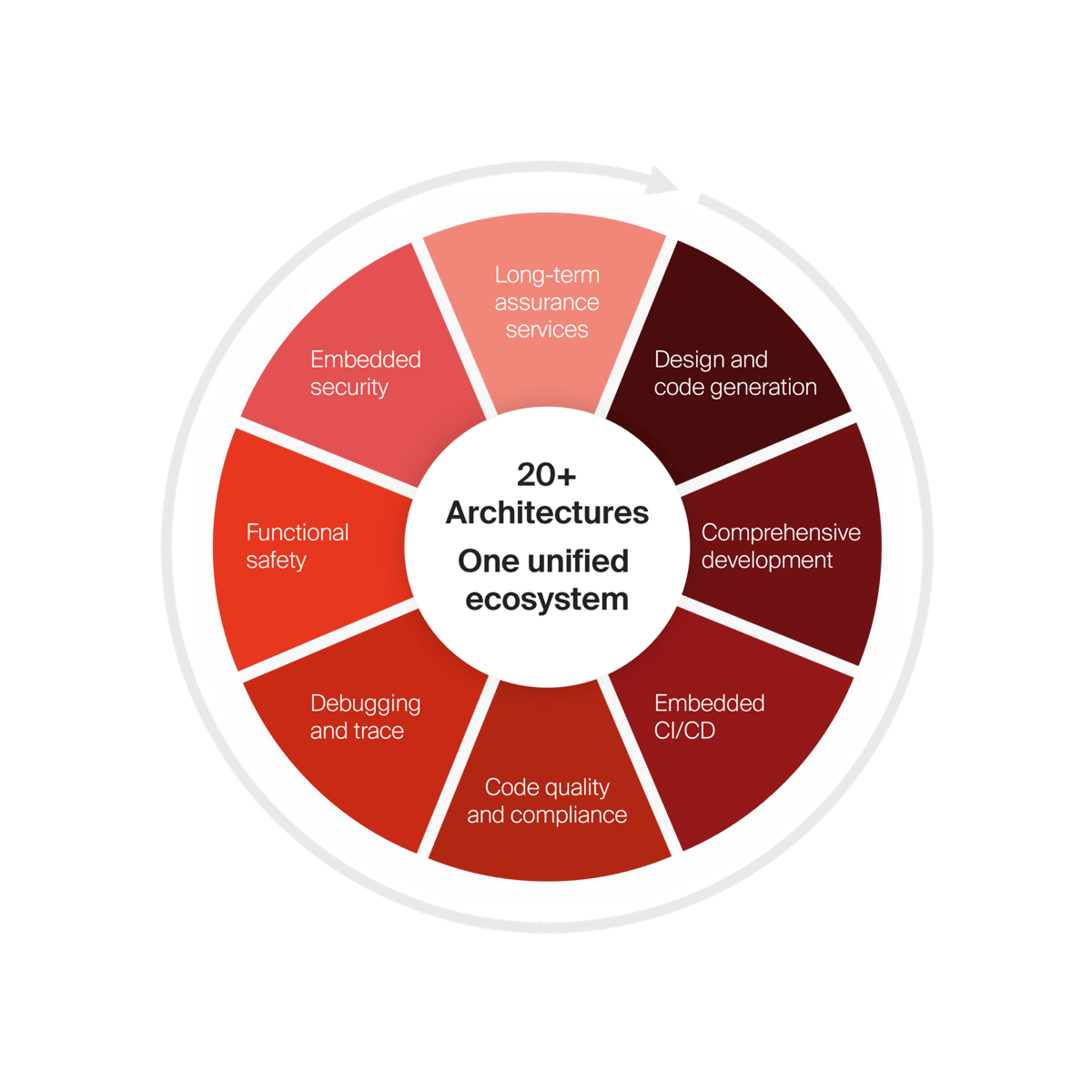
IAR embedded development platform
Scale development operations with freedom and flexibility, accelerate innovation with code confidence and simplify compliance while strengthening security.
With our platform you get access to everything.
